Common fluorescent label has unintended impact on health of zebrafish
By Andrea Brumwell
March 8, 2018
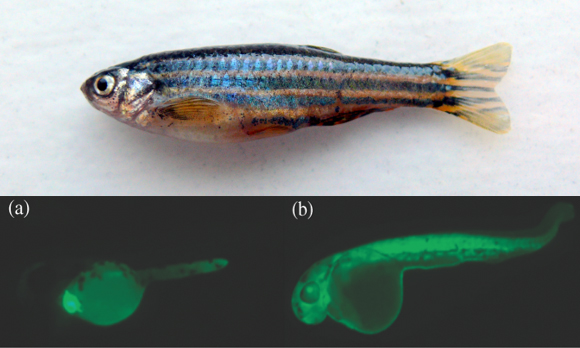
Zebrafish are a common study animal in labs. Genetically altered individuals are typically labelled with a fluorescent protein gene so they can be easily identified [Photo credit: (top) Pogrebnoj-Alexandroff CC BY-SA 3.0; (bottom) Todd Gillis]
A glowing protein used as a marker to identify transgenic zebrafish may have unintended consequences on the health of these important study animals, suggests a new study by researchers in the Departments of Integrative Biology and Molecular and Cellular Biology.
A team of researchers including graduate student Sean Avey, and Profs. Todd Gillis and John Dawson, has discovered that green fluorescent protein (GFP), a glowing molecular label, has detrimental effects on cardiac function and swimming ability when expressed throughout the body of zebrafish.
“GFP is meant to be an innocuous marker that helps researchers identify fish that have been genetically altered for research,” says Gillis. “But no one has really examined if this marker gene has unintended effects on the animal that might impact how we interpret our research results.”
The Gillis and Dawson labs use zebrafish to study the mechanisms underlying normal cardiac function and how these go wrong in heart disease. Zebrafish make good models for a number of human diseases because their genes are easy to manipulate and share many similarities with their human counterparts. Researchers inject the GFP gene into zebrafish embryos along with the disease-related gene that they are interested in studying. Because zebrafish embryos are transparent, GFP acts as a shining beacon, allowing researchers to easily see which fish successfully incorporated the gene of interest. It also tells them where in the body the two introduced genes are expressed.
But Gillis says that studies using this technique do not always use the proper controls. This means that when changes are seen in the transgenic fish, it is not clear whether the changes are due to the GFP or the gene being studied.
To find out, the researchers created two types of zebrafish, one where GFP was expressed in the heart (image a), and another where a human cardiac actin gene was expressed in the heart and GFP was expressed throughout the entire body (image b). Cardiac actin is a protein that is critical to enabling the heart to contract and pump blood; it was included in the study because it will be the control for any future studies examining the effects of actin mutants on zebrafish health.
The team then tested the heart function, swimming abilities, and metabolic rate of both types of fish compared to their unaltered counterparts.
Zebrafish with human actin in the heart and GFP expressed throughout the body consumed nearly twice as much energy during swimming. Gillis and colleagues believe that GFP interferes with the function of swimming muscles, making these fish less efficient swimmers.
The heart rate during embryonic development of the same fish was lower compared to the controls and there was also a higher mortality rate, indicating that whole-body GFP expression had a negative impact on the overall health of the fish.
The findings have important implications for studies that use GFP as a molecular marker, says Gillis. “We now know how GFP expression alone is affecting the animals and that we need to take this into account when looking at consequences of further manipulation.”
Matiyo Ojehomon also contributed to the project. Funding for this project was provided by the Natural Sciences and Engineering Research Council and the Heart and Stroke Foundation of Canada.
Read the full article in the Journal of Fish Biology.
Read about other CBS Research Highlights.