How to Improve Drug Production: An Unbe-leaf-able Story
By Harshina Brijlall
14 June 2021

Tobacco plants used to produce drugs (photo by N. Prudhomme)
As Canadians eagerly await their COVID-19 vaccine and an end to the pandemic, the need for rapid and low-cost drug production has never been more front and centre. This makes the work of researchers like Dr. Jennifer Geddes-McAlister, an assistant professor in the Department of Molecular and Cellular Biology, more timely than ever. The microbiologist and proteomics (study of proteins) expert is helping to improve a new form of drug production: molecular farming using plants.
“There are many benefits of producing pharmaceutical proteins in plants,” explains Geddes-McAlister.
“It is low cost, does not require animals, and can be rapidly deployed when new therapies are urgently needed.”
A popular approach to molecular farming involves the use of Agrobacterium tumefaciens, a naturally occurring bacterial plant pathogen that injects part of its DNA directly into plant cells during infection. It is a completely natural example of one species delivering genetic material into another.
Decades ago, scientists learned to harness this gene transferring ability to create the first genetically modified crops. Today, A. tumefaciens is being used to create plants that produce high value proteins such as pharmaceuticals.
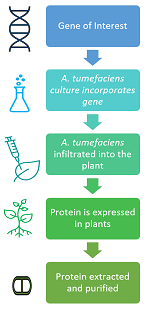
The method involves five basic steps (see schematic at right). First, the gene that codes for the desired protein is incorporated into the bacteria, which is then mass cultured. This is followed by a process called “agroinfiltration”, where the bacteria are applied to the plant and transfer the gene of interest to plant cells. The plant then becomes a miniature drug factory, rapidly producing the protein of interest as it grows. Finally, the plant is harvested, and the proteins extracted and purified for use.
Plantform Corporation is a local biotechnology company using plants to produce antibodies and other pharmaceutical proteins. They recently reached out to the Geddes-McAlister lab to help find ways to improve the agroinfiltration processes.
“Plant-based protein drug production is a fairly new and exciting area of study that is rapidly developing, and the industry is still figuring out the best ways to do it on an industrial scale,” explains Geddes-McAlister.

Illustration of a shake flask (left)
and bioreactor (right)
For example, molecular farming with A. tumefaciens requires growing large quantities of the bacteria. This can be done in simple vessels called shake flasks, but this limits the volume of bacteria that can be generated. It also offers little control over the growing conditions. In contrast, a bioreactor can hold up to 100 litres and allows much greater control of the bacteria’s growing conditions. But it isn’t clear how these different culture techniques can affect the bacteria’s ability to infect plant tissue. And when it comes to drug production, consistency at all stages of the production pipeline is critical.
This question led Geddes-McAlister and graduate student Nick Prudhomme to analyze and compare almost 3,000 different proteins produced by A. tumefaciens grown in both shaker flasks and bioreactors.
Proteins are essential to all cellular processes, and they can tell a unique story about the status of an organism at any given time. By comparing the bacteria’s protein profile when cultured in flasks versus bioreactors, Prudhomme and Geddes-McAlister could identify key differences in bacterial function under each production method.
The results showed that there were 25 proteins produced in much higher abundance during bioreactor growth, while another 12 proteins were produced in higher quantities during flask growth. With many of these proteins linked to key metabolic pathways, the study clearly showed that culture method affects important functions in A. tumefaciens. From a drug production standpoint, this could have significant implications at the plant infection stage.
In a second study, Prudhomme and Geddes-McAlister applied the power of proteomics to another step in the production process. This time, they examined how the protein profiles of bacteria changed over time while in the agroinfiltration solution, which lacks nutrients for the bacteria to feed on. This fasting period could be short or last several hours, depending on when the plants get added to the pipeline.
Quite unexpectedly, the researchers found that prolonged exposure to the agroinfiltration solution (up to 24 hours) has a beneficial effect on the bacteria’s ability to infect the plants. Proteomic analysis revealed that the bacteria underwent a cellular “remodelling” that was linked to increased motility (movement) and other characteristics associated with enhanced host infection.
“A longer exposure period to the agroinfiltration solution seems to help ‘prime’ the bacteria in preparation to invade the host (plant) cell,” says Geddes-McAlister. “And ultimately, the more infective the bacteria are, the greater the production of the target drug.”
The Geddes-McAlister lab’s next steps involve investigating the host plant’s protein profiles when infected with the bacteria and learning how this impacts target protein production.
“If we can contribute to the production of drugs in a cost-effective way and help treat a variety of diseases, that's a direct and very rewarding application of our research.”
Bioreactors and training were provided by the lab of Dr. Emma Allen-Vercoe, Department of Molecular and Cellular Biology. Funding for the study was provided by the the Natural Sciences and Engineering Research Council.
Read the about both studies in the Canadian Journal of Microbiology: article #1, article #2
Read about other CBS Research Highlights.